

Features of Gastromotal
Indications for the use of Gastromotal
Gastromotal is a ¹³C-Octanoic Acid breath test to determine the rate at which solid food passes from the stomach into the small intestine. Gastric motility is affected in a number of clinical conditions including diabetes, non-ulcer dyspepsia, GORD and some post-operative conditions. The recent development of drugs affecting gastric motility has increased the level of interest in the process.
INFAI has completed clinical trials for this test and is currently applying for an application for a Europe-wide Marketing Authorisation. The kit is currently available for use on a named patient basis and for research applications providing the appropriate ethical permissions and licence exemptions are in force.
Test Principle and Protocol of Gastromotal
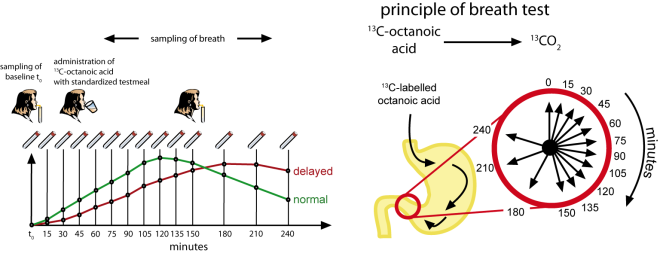
The techniques currently available to determine gastric emptying (scintigraphy and ultrasound imaging) have significant deficiencies, so a new and better test will be of considerable value. Ghoos et al (Gastroenterology, Vol 104, 1640-1647, 1993) described a ¹³C-Octanoic Acid breath test in which the substrate is incorporated into an egg testmeal and the appearance of 13CO2 in the breath is monitored. ¹³C-Octanoic Acid occurs as part of naturally occurring components in foods such as butter so no adverse effects are to be expected. Mathematical analysis of the excretion curve using a procedure developed at INFAI permits the gastric emptying rate to be determined. The test provides a non-invasive approach to the identification of patients with gastric motility problems and the evaluation of promotility drugs.
After an overnight fast the patient ingests ¹³C-Octanoic Acid mixed with raw egg yolk, cooked and served with bread, butter and 150 ml of coffee or tea. In this form the ¹³C-Octanoic Acid is not released until it reaches the small intestine, where it is absorbed and rapidly metabolised in the liver. The 13CO2 produced appears in the breath almost immediately and is sampled by asking the patient to blow through a straw into glass tubes, which are sealed and stored for analysis. Two breath samples are collected immediately before the test, 10 breath samples at 15 minute intervals after the test meal and a further three at 30 minute intervals covering a total measurement period of 240 minutes. The tubes are sent for 13CO2-analysis at a qualified laboratory and the excretion curve is mathematically analysed to differentiate normal and reduced gastric motility. Two parameters are calculated and reported (Gastric Emptying Coefficient and Gastric Half Emptying Time) using a mathematical procedure and software package developed by INFAI.
Since the test accurately defines the time at which solid material arrives in the small intestine, the preparation of the testmeal is crucial to the efficacy of the test. If the testmeal is incorrectly prepared the label may be washed out of the stomach in the liquid phase which would mask reduced gastric motility.
Ongoing study for Gastromotal
Study: Gastromotal; Eudra CT Number: 2011-002782-38
Gastromotal 1-¹³C-Caprylic Acid breath test in the diagnosis and evaluation of therapeutic outcome in patients with dyspeptic symptoms and delayed emptying. Sponsor: INFAI
Gastromotal Patent
Publications